- Tips
- technology
- Frequently Asked Questions
- Tests
- mAh capacity
- Rated Capacity
- comparison
- everActive
- Batteries vs rechargeable batteries
- Accumulated energy
- Durability of rechargeable batteries
- Efficiency of rechargeable batteries
- battery voltage
- LR03 AAA
- LR6 AA
- eneloop
- AG13 LR1154 LR44
- CR 2032
- Delta V
- Charge Cycles
- internal resistance
- charge level
- memory effect
- accredited test
- SR44 357
- Hearing Batteries 675
- SR626 377
- Watch Batteries
- Polarity
- Mah
- passivation
- LS 14250
- LS 14500
Does fast charging harm Ni-MH batteries? How to properly charge nickel-metal hydride batteries?
Many of our customers still believe that the slower we charge a battery, the better it is for its condition and life. Unfortunately, it is very easy to unknowingly destroy your batteries. If we charge new AA batteries with a current of 200-300 mA, we certainly do not use their full potential, and we can even destroy them. The basic issue is what too fast or too slow charging actually means.
It is understandable that we do not want to lead to too fast wear of the batteries by charging them too quickly.
However, you need to be aware of what fast charging actually means. Years ago, there was a fashion for 15, 30, 45-minute chargers. These were actually fast chargers.
These chargers had a charging current of 2A and more for each installed cell.
In general, in the case of Ni-MH technology with the battery, nothing bad happens as long as the temperature does not regularly exceed 50 degrees Celsius during charging. This limit in these fast chargers was quite often crossed, and the chargers themselves practically completely disappeared from the market.
Ni-MH technology is considered to be generally safe, m.in. due to the non-invasive possibility of dissipating (transferring) excess energy in the form of heat - as long as the heat is kept in check, there should be no harm to the battery.
So to sum up this thread - for a new 2000 mAh battery, the current of 1A (charging time of just over 2 hours) is not yet excessively high, real problems with heat begin to appear only at values of 1C (where C is the capacity of the battery), i.e. 2000mA for a 2000mAh battery, 2600mA for a 2600mAh battery, etc.
I already know when we are talking about fast charging or high charging current, but I don't understand what the problems with slow charging or too low charging current come from?
Charging of a typical Ni-MH battery ends when the voltage on the battery reaches its maximum value (this value can be different for each battery and even for each charging cycle, as it depends on many factors, such as temperature or charging current), and then begins to slowly decrease. The problem is that at 0.1C (e.g. 190 mA for a 1900 mAh battery) this phenomenon will never occur. At 0.2C (380 mA for a 1900 mAh battery) the effect will still be minimal. We will show this below in the respective charts.
After all, on my 1900 mAh battery, the manufacturer clearly writes "Standard charge: 190 mA for 16h".
The "standard charge" notation in the form we see on rechargeable batteries (regardless of the manufacturer) is a requirement of IEC/EN standards - it applies to charging a previously discharged battery to 1.0V, with 0.1C current for a period of 16 hours - without the participation of any automation, voltage control, etc.
In the era of advanced chargers, this is a largely nonsensical provision / requirement, having nothing to do with a real recommendation. However, as a rule, it must be placed on the battery, it specifies the conditions under which the manufacturer guarantees that the minimum capacity of the battery will be achieved.
What are the characteristics of charging the battery with currents of 0.1C, 0.2C, 0.5C and 1C? What are the consequences of such charging?
We illustrate this on the example of the everActive Silver Line AA R6 2000 battery, with a minimum capacity of 1900 mAh.
1. Charging with 0.1C, i.e. 190 mA in the case of a 1900 mAh battery.

It is assumed that with such a low charging current, charging should take about 14-16 hours. The only determinant of a full charge here is time and the fact that the voltage on the battery has been stabilized at some point, although it is still slowly increasing.
As you can see, the voltage on the battery, even after 16 hours, continues to increase, despite the fact that the battery has already been fully charged. In such conditions, any automatic charger may have a problem with the correct assessment of full charge. As a result, the battery is very often undercharged or overcharged - in the case of regular overcharging, we lead to faster wear of our cells. In the case of regular undercharging, our cell may be affected by the so-called lazy battery effect and we may have problems with its full use.

2. 0.2C charging, which is 380 mA for a 1900 mAh battery.
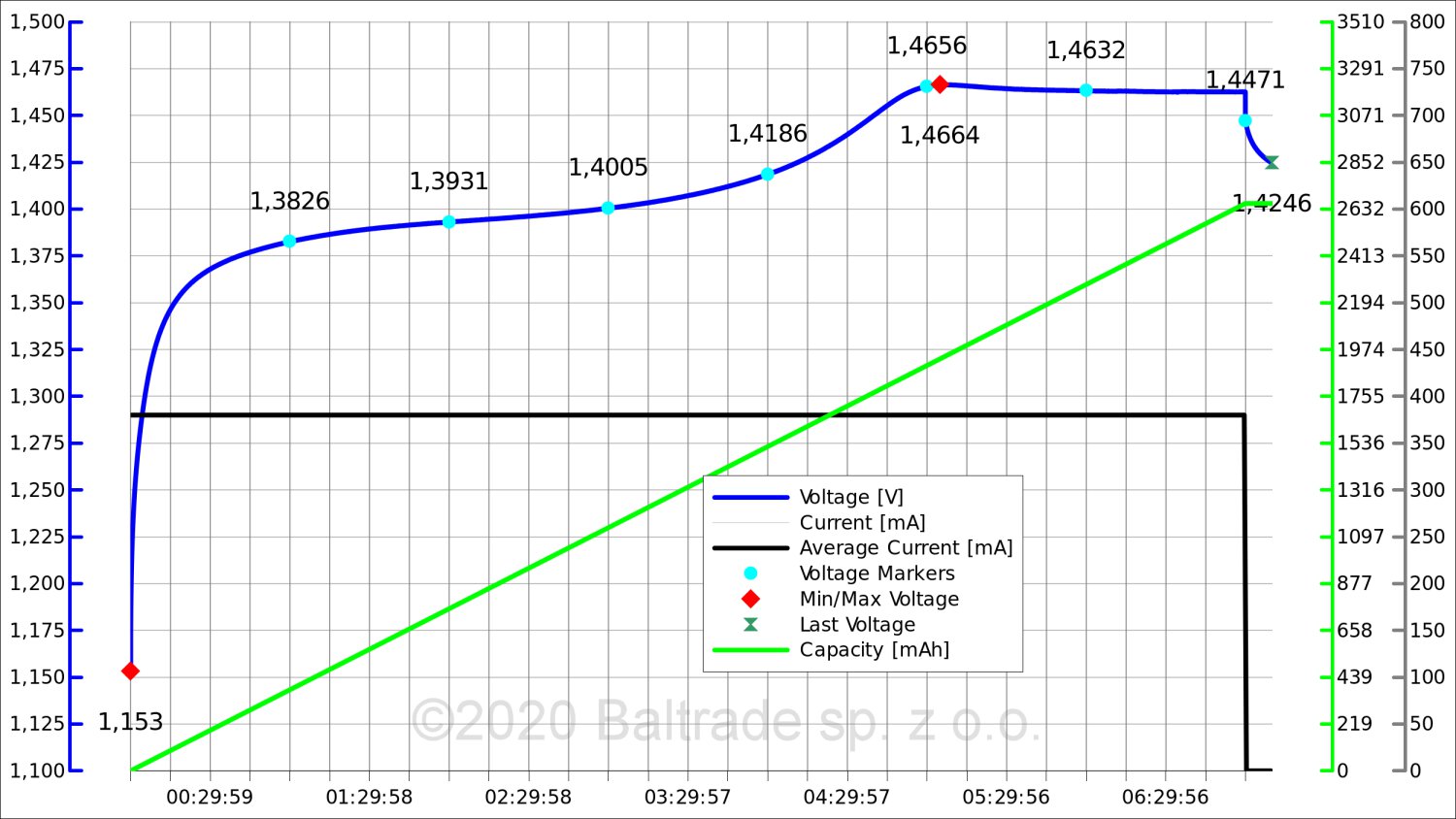
The battery was charged in about 6 hours. As we will show below, however, this is a very small change.
The voltage drop at 0.2C was only 3 mV. Good microprocessor chargers are able to catch differences of 2-3 mV and charging in such conditions has a chance to end properly. However, due to the very small nature of the voltage change (drop) in the last phase, this method of charging still carries the risk of incorrect assessment of the charge by the charger.
If our charger allows us to choose the charging current, then the value of 0.2C should be treated as the minimum.

3. 0.5C charging, which is 950 mA for a 1900 mAh battery.
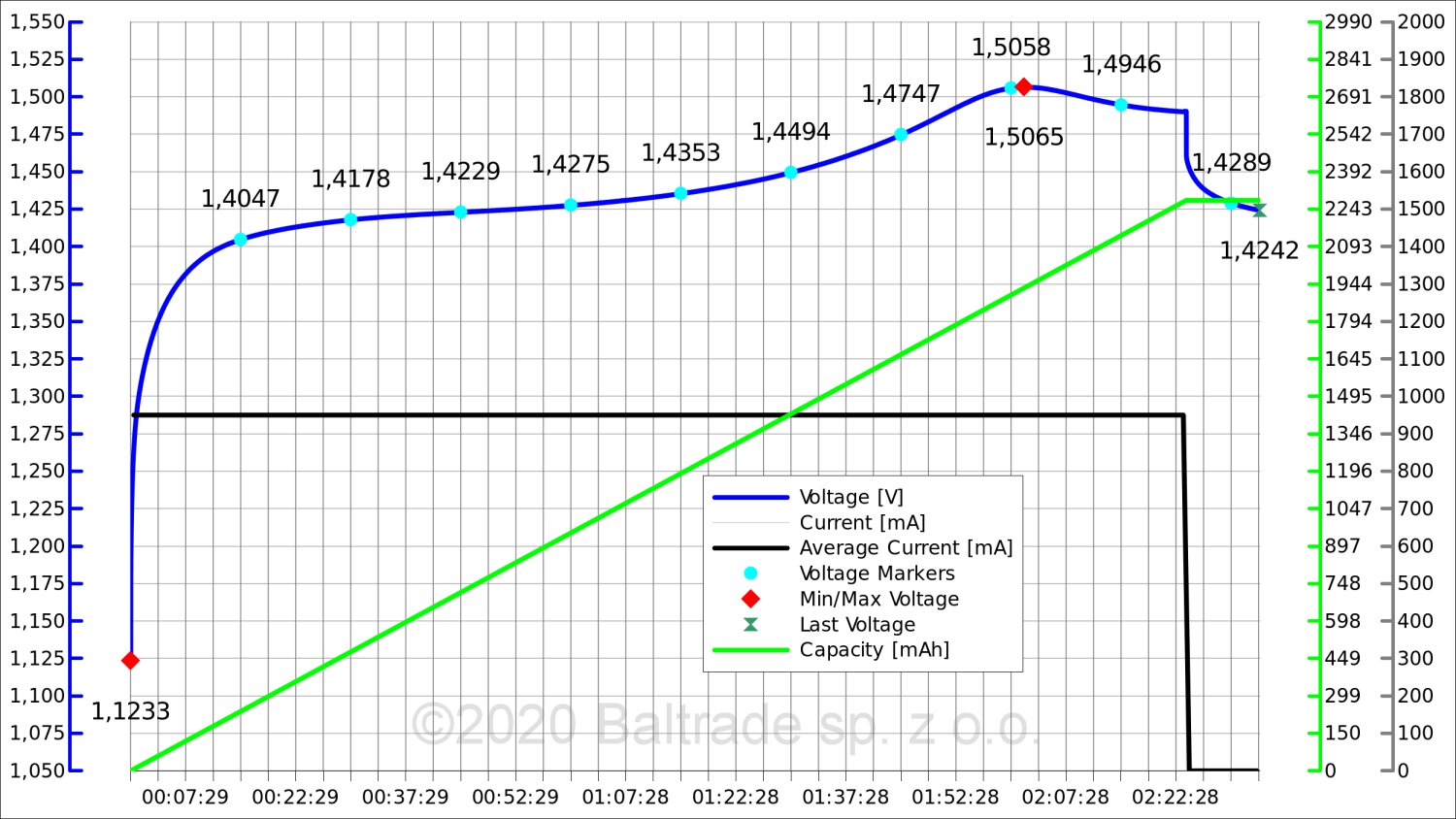
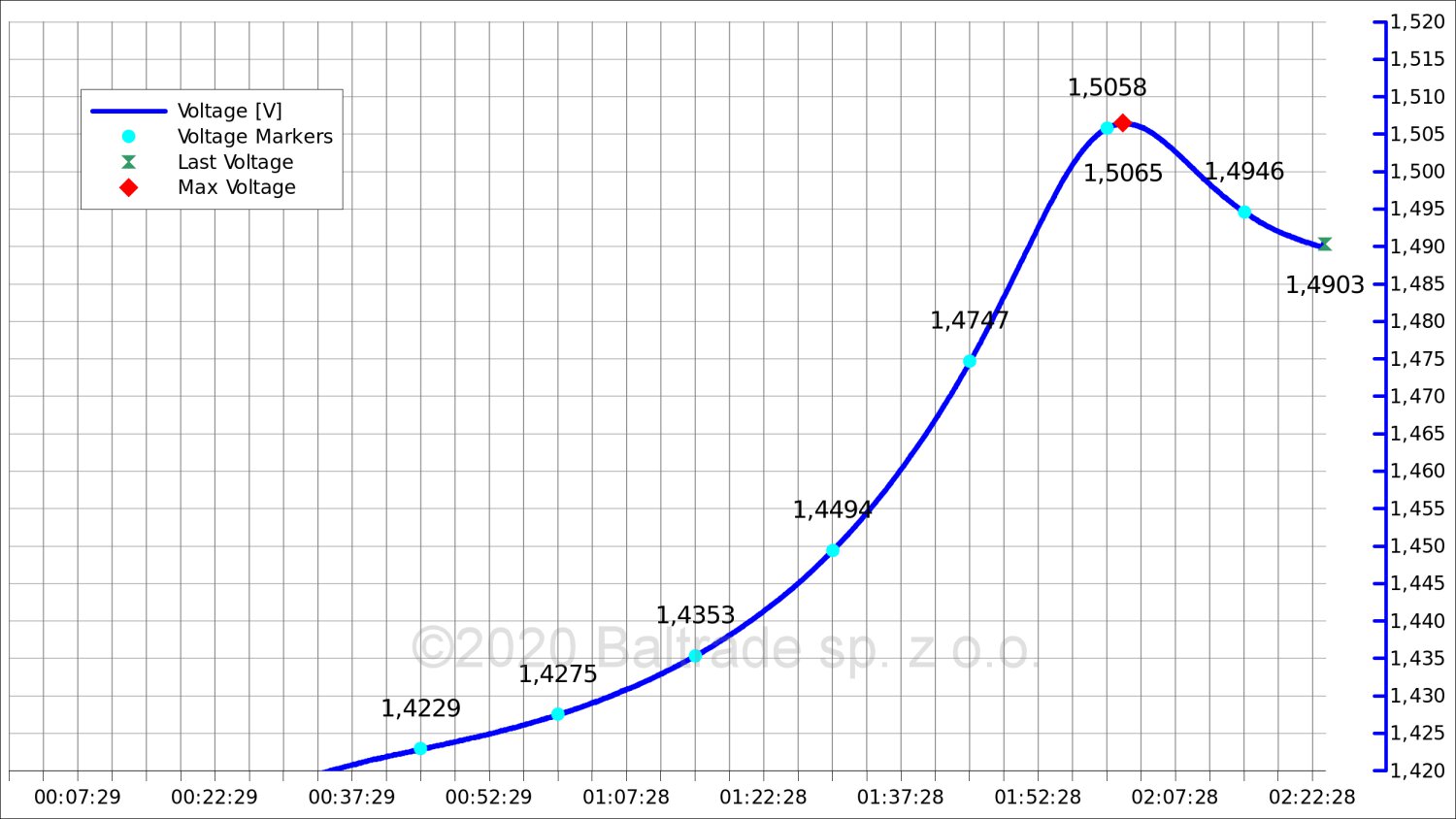
Charging took just over 2 hours. This time you can already see a rather characteristic voltage hump at the end of charging. The change is already clearly visible.
The voltage drop at 0.5C was 15 mV here. Most chargers should no longer have a problem with correctly assessing the moment of full charge.
However, with a charging current of 1000 mA in compact, popular chargers, there may be a thermal problem related to the heat generation of the charger itself. Temperature changes "from the outside" can effectively disrupt the charging process of the battery, which sometimes also causes it to heat up excessively.
Analyzing the above graphs, we already know why it is often assumed that the optimal charging current is a value in the range of 0.2-0.5C.
4. 1C charging, which is 1900 mA for a 1900 mAh battery.
Charging was completed in just over an hour. The change in voltage is very visible, its drop is even steeper. As we can see, the higher the charging current, the easier it is to notice the voltage drop on the battery at the end of charging.

This time the voltage drop was nearly 20 mV. The difference was relatively large, but the battery was already clearly warm at the end of charging.
If we add to this the possible problems with heating up the charger itself, we may have a problem with maintaining a sufficiently low temperature of the cell, which may end up with significant overcharging and overheating of the battery.
With currents of 1C, active cooling of the charger or additional, sensitive temperature sensors are often recommended.
With a charging current of 1C and higher, it also happens that the battery is slightly undercharged - charging may be completed prematurely due to the rapid increase in temperature.

In summary, currents of 0.1C (p.1) are often too low
Currents of 0.2C-0.5C (p.2,p.3) are considered to be the most optimal, allowing automatic chargers to correctly assess when the battery is fully charged. With these currents, there is also the lowest risk of overheating the battery.
Currents 0.5C-1C (p.4) - at these values, the temperature of the environment and the charger itself during charging is important - any sudden changes in temperature can disrupt the charging process and lead to dangerous heating of the battery. Such high charging currents will also heat up batteries that are already partially worn out and worn out much more.
Currents above 1C - this is what we call too high charging current. We recommend that you avoid regular use of any 15-30 minute chargers. Although these chargers often have additional cooling, they often have problems with precise charging of the batteries and can lead to faster battery wear. There is also often a problem with charging partially used batteries.
Author: Michał Seredziński
Copying the content of the text or its part without the consent of a representative of Baltrade sp. z o.o. is prohibited.
-
Witam ! Dziękuję serdecznie za obszerne i profesjonalne informacje*Bardzo mi pomogły w przygotowaniu do powrotu, do używania akyumulatorków NiMh*Pozdrawiam !
-
najbardziej zaciekawiło mnie to że 1,9Ah aku przyjął (odczytane z wykresu):
0,1C - 3,1Ah
0,2C - 2,6Ah
0,5C - 2,2Ah
1,0C - 2,2Ah
więc zasadnicze pytanie brzmi dla jakiego prądu rzeczywista ilość zgromadzonej energii była największa?-
W każdym z tych przypadków rzeczywista ilość energii przyjęta przez akumulator była niemal identyczna, choć przy najwyższych prądach ładowania jest minimalnie (o kilka procent) niższa.
Akumulator Ni-MH będzie "przyjmował" tak długo energię jak długo będziemy ją do niego dostarczali. Ta chemia ma tą zaletę, że nadwyżki energii, której nie jest w stanie przyjąć wytraca w formie ciepła - o ile tego ciepła nie ma zbyt dużo, wówczas jest to proces dość bezpieczny, z niewielkim wpływem na żywotność samego ogniwa.
Niemniej zauważona obserwacja jest zgodna z praktyką - ogniwo 1,9Ah przy 0,1C zgodnie z odpowiednią normą IEC/PN-EN ładujemy do 3040 mAh - bez żadnej automatyki, licząc się z tym, że akumulator zostanie przeładowany - jednak z uwagi na niski prąd ładowania, ilość wydzielonego ciepła na akumulatorze będzie niewielka.
Przy 0,2C mamy jeszcze teoretycznie 2 wyjścia - albo ładujemy ogniwo przez ok. 6,5h bez żadnej automatyki - ogniwo 1900 mAh jest wtedy ładowane do ok. 2500 mAh. Tutaj już ilość ciepła będzie istotnie wyższa, mimo mniejszego przeładowania ogniwa.
Dlatego przy prądach 0,2C i wyższych potrzebna jest już zwykle dodatkowa automatyka, gdzie ładowarka stara się możliwie szybko wykryć moment pełnego naładowania ogniwa. Ilość władowanych mAh do pustego akumulatora stanowi zwykle wartość 105-120% jego faktycznej pojemności.
Teraz im wyższy prąd ładowania tym przeładowanie ogniwa liczone w mAh jest zwykle niższe - mimo to temperatura końcowa ładowania będzie wyższa wraz z wyższym prądem ładowania.
Przy prądach rzędu 2C zwykle ładowarka nie jest już w stanie bezpiecznie dostarczyć do akumulatora nawet 100% jego pojemności liczonej w mAh (temperatura jest już wysoka) - i taki akumulator może być niedoładowany.
-
-
Jak zwykle pełen profesjonalizm. Też mi miło odświeżyć sobie dobrze zaprezentowane wiadomości. Pozdrawiam.
-
Przepięknie opisany temat ładowania niklów, wiedzę na temat ładowania zdobyłem 15 lat temu, przypadkowo natrafiłem na tą stronę zobaczyłem 2 wykresy i przeczytałem całość w celu "odświeżenia". Używam liitokali 600 do przeróżnych ogniw litowych i niklów, ale nigdy bym nie wpadł na pomysł ładować niklowego prądem 1C ( 2 ampery) o.O przecież ładowarka go za szybko odetnie i będzie tylko w 3/4 naładowany swojej całej pojemności. Jeżeli już ktoś ma dany sprzęt na baterie AA lub AAA czy to lampa błyskowa czy to pilot, zegar, pad, diskman, postanowił używać akumulatorków niklowych, to niech nie mówi że "NIE MA CZASU" na ładowanie prądem 250-500 mA :) teraz 99% akumulatorków AA ma pojemność 2500 mAh, przyczyniając się do wzoru prądu 0,1C to ładowanie wynosi 250mA i takie też zalecam każdemu stosować w celu naładowania w pełni swojego aku, tzw. prąd dziesięciogodzinny, a w najgorszym przypadku używać 500mA.
-
Panie Michale, to bardzo dobry artykuł wyjaśniający jak powinno się ładować akumulatory niklowo wodorkowe i niklowo kadmowe. Szkoda, że taka rzetelna wiedza nie jest przekazywana powszechnie. I bez głupich docinków jak na elektrodzie, gdzie 75% wątku to jałowa dyskusja, kpiny i kłótnie.
-
Prosto, jasno i na temat. Brawo.