- Tips
- technology
- Frequently Asked Questions
- Tests
- mAh capacity
- Rated Capacity
- comparison
- everActive
- Batteries vs rechargeable batteries
- Accumulated energy
- Durability of rechargeable batteries
- Efficiency of rechargeable batteries
- battery voltage
- LR03 AAA
- LR6 AA
- eneloop
- AG13 LR1154 LR44
- CR 2032
- Delta V
- Charge Cycles
- internal resistance
- charge level
- memory effect
- accredited test
- SR44 357
- Hearing Batteries 675
- SR626 377
- Watch Batteries
- Polarity
- Mah
- passivation
- LS 14250
- LS 14500
How does the battery work?
2019-06-19
Looking at the battery, we can see that it has two poles - one positive, marked "+", the other negative - marked "-". In the case of typical cylindrical batteries, such as R6/AA or R14/C (used, for example, to power flashlights or toys), the terminals are the ends of the battery. In car batteries, the poles are heavy lead terminals.
Electrons accumulate on the negative pole of the battery. If we connect the negative and positive terminals with a wire, the electrons will move as quickly as possible from the negative to the positive terminal - the battery will run out very quickly (in addition, we advise against this type of experiments due to the dangers associated with them - never short-circuit the battery in this way "for a short time"!). Under normal conditions, we connect a load to the battery with a cable - a light bulb, motor, or an electronic circuit, such as a radio.
Inside the battery, a reaction takes place that produces free electrons. The speed at which electrons are released as a result of this reaction (internal resistance - resistance - of the battery) obviously limits the number of electrons that can flow between the poles. Electrons must flow from the battery through the wire and the load, from the negative to the positive terminal, for a chemical reaction to take place that will release even more of them. For this reason, we can leave the battery unused on the shelf for e.g. a year, and then continue to use it without any problems - as long as the electrons do not flow from the negative to the positive terminal, the chemical reaction does not take place. The moment the poles are connected - the reaction begins.
Chemical reactions in batteries
One of the simplest batteries is the zinc-carbon battery. By looking at the reactions taking place inside it, we can more easily understand the general principle of operation of all batteries. Imagine that we have a jar of sulfuric acid (H2SO4). If you place a zinc rod in it, the corrosive acid will immediately start to dissolve it. We'll see hydrogen bubbles collecting on the surface of the zinc, and both the rod and the acid will start to heat up.Here's what happens:
- acid molecules decay into three ions: two H+ ions and one SO4-ion
- zinc atoms on the surface of the rod lose two electrons (2e-) and become Zn++ ions
- Zn++ ions combine with SO4-- ions to form ZnSO4, which dissolves in acid
- electrons from zinc atoms combine with H+ ions and form H2 molecules (hydrogen gas)
- The electrons will start to move along the wire and combine with the hydrogen on the carbon rod, from which hydrogen bubbles will now also start to evolve
- heat release will be significantly reduced; Using the electricity flowing through the wire now, we could, for example, power a light bulb - and measure the resulting voltage and current flowing through the wire - some of the heat energy was converted into the movement of electrons.
The batteries we know work on the same principle. They differ in the types of metals and electrolytes used, but they all work thanks to the same phenomenon - electrons flowing from one pole to the other. Depending on the components used, the characteristic voltage of such a battery also changes. Let's follow the example of a typical lead car battery:
- The battery contains one plate made of lead and another made of lead dioxide, both immersed in an electrolyte of highly concentrated sulfuric acid
- lead combines with SO4 to form PbSO4 and one free electron
- lead dioxide, hydrogen ions and SO4 ions, and electrons from the lead plate, form PbSO4 and water on the plate from lead dioxide over time, the two plates become coated with PbSO4 and the water mixes with the acid; the characteristic voltage is about 2V - so, by connecting 6 cells in series, we get a battery of cells with a voltage of 12V
- zinc-carbon batteries - so popular that they are sometimes called "ordinary"; are the most commonly used batteries, in sizes such as R6/AA, R14/C, R20/D; the electrodes are made of zinc and
- carbon, with an acid paste sandwiched between them, serving as an electrolyte
- Batteries A
Cell/Battery Bonding
In the previous considerations, we used the words "battery" and "cell" interchangeably. This is in line with the tendency prevailing in everyday language. However, from a technical point of view, the words "battery" and "cell" have quite different meanings. And so, "cell" means a single power source, e.g. such as the jar of acid described at the beginning and two rods connected with a wire (or, for example, an R6/AA "finger"). A "battery" is a set of connected cells (such as a 3R12 battery, consisting of three cells in one housing, connected in series). It is in this sense that we shall use these two terms in the following part of this text.In most devices, we tend not to use a single cell. Instead, we connect several of them - either in series, for higher voltage, or in parallel, for higher currents. In series connection, we obtain the sum of the voltages of the connected cells; in parallel connection - the sum of currents obtained from the component cells.
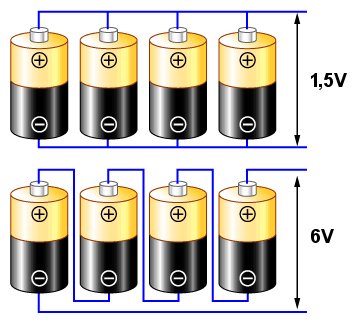
The connection as in the upper diagram is called parallel. If we assume that each cell has a characteristic voltage of 1.5V (like a typical single zinc-carbon or alkaline cell), then the voltage obtained at the end terminals (indicated by arrows) will still be 1.5V, but the obtained current will be four times higher than that obtained from a single cell.
The connection as in the bottom diagram is called serial. In this case, the voltages from the individual cells add up, giving a voltage of 6V between the terminals.
When buying a battery or cell, you can usually read on the packaging what its voltage is - sometimes also its capacity. For example, typical rechargeable batteries used in digital cameras have a voltage of 1.2V and a capacity of 2000mAh. A capacity of 2000mAh (mAh is an abbreviation for milliampere-hour) means that, theoretically, such a battery is able to provide current of 2000mA (2000 milliamperes, i.e. 2 amps) for an hour, current of 1A for two hours, current of 100mA for 20 hours, etc. However, cells usually do not behave so linearly. First, each battery has a maximum current that it is capable of delivering. And so, a 500mAh battery will not be able to provide 30A of current for a second, because there is no way for the chemical reactions taking place inside the battery to provide so many electrons in such a short time. Secondly, at high currents, the cells usually heat up a lot, which wastes a lot of their energy. Third, many of the chemistry systems used in batteries last shorter (or longer!) with very low power consumption. Nevertheless, the capacitance measured in ampere-hours gives a good idea of how long a given cell will last at a certain current consumption, under typical operating conditions.
Copyright © Baltrade
Add a comment